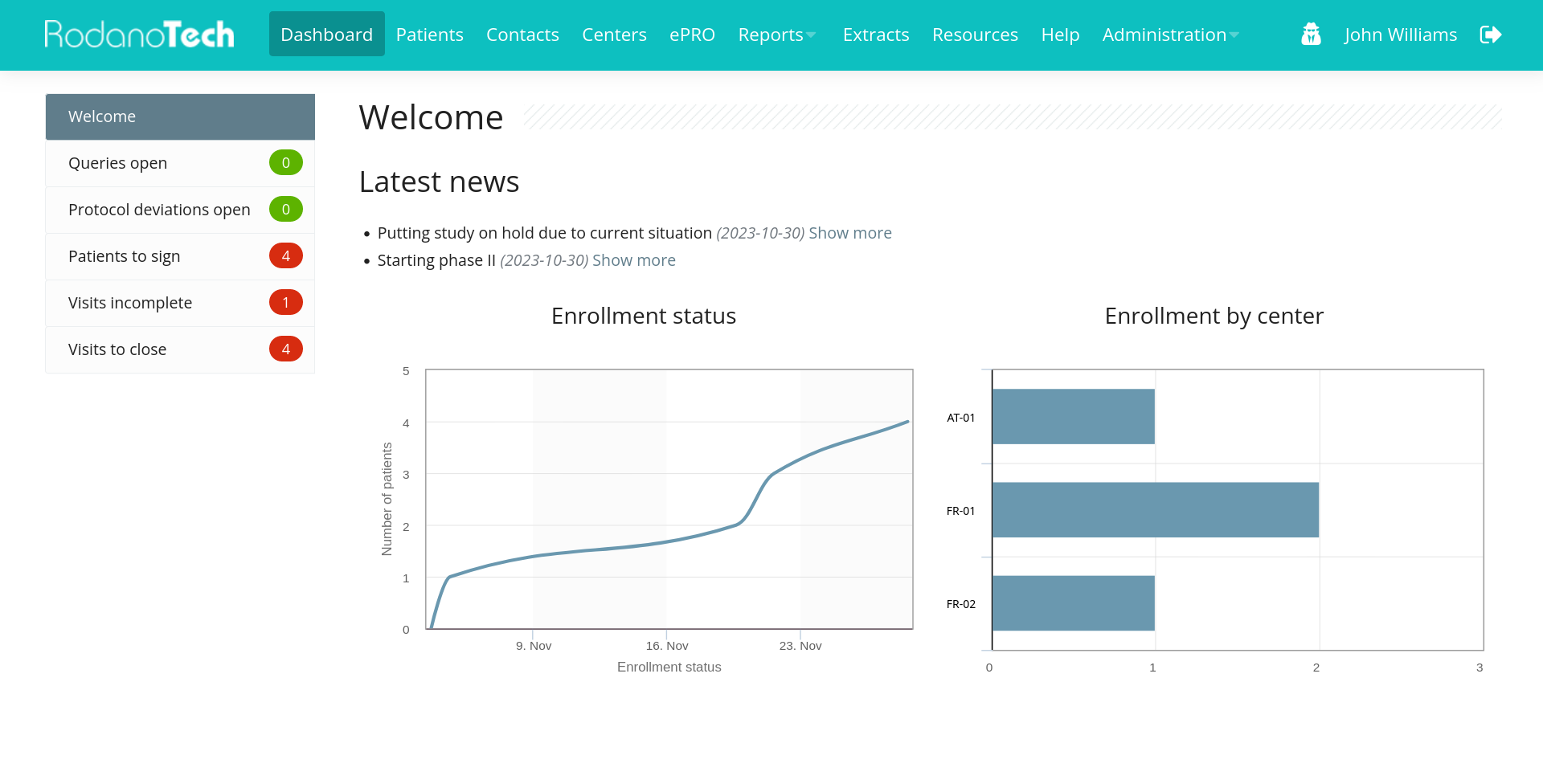
Rodano is an open source EDC platform for all types of clinical trials, cohorts or registries

Why Rodano?
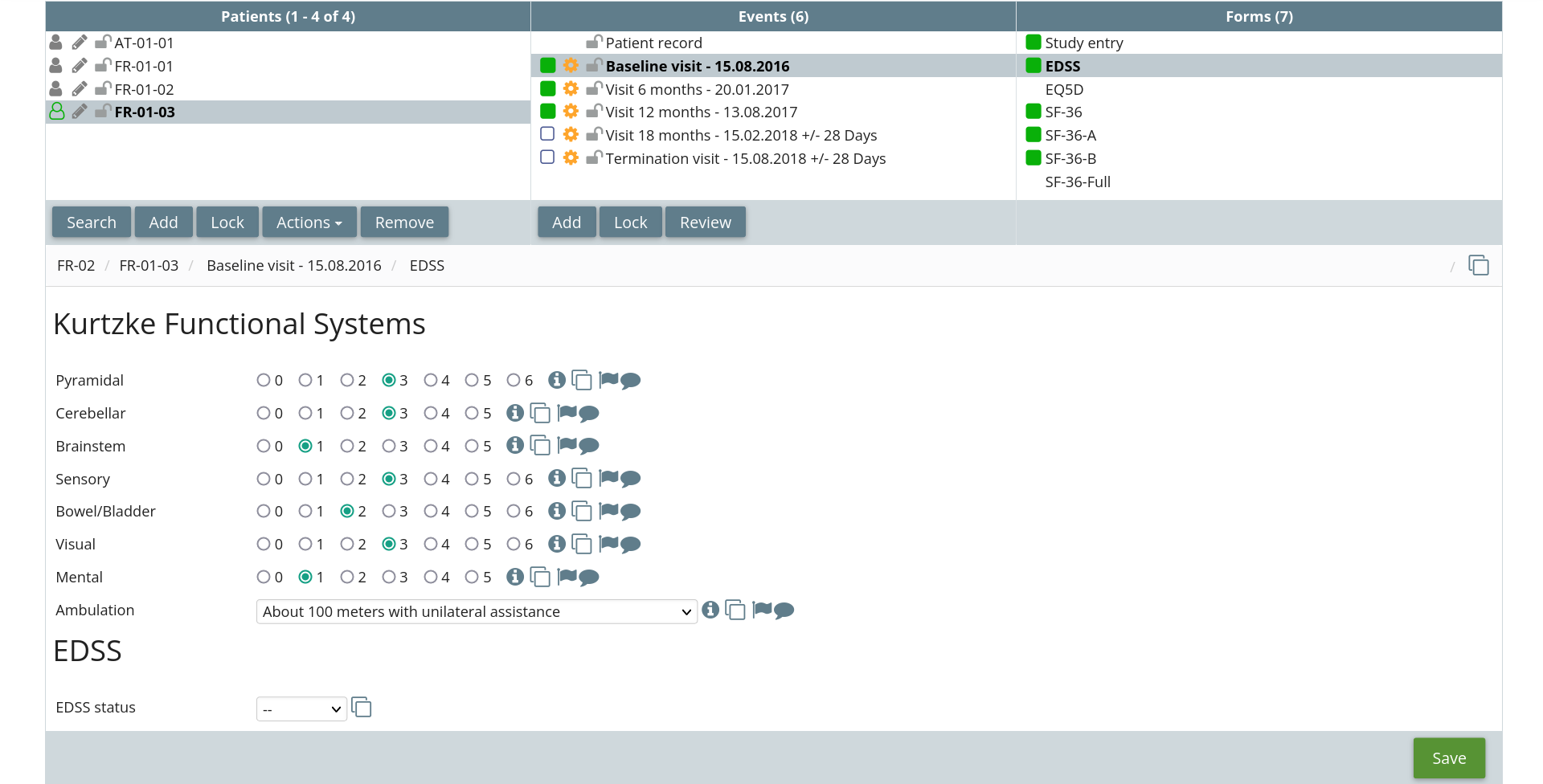
Fully featured
All the features you would expect from a modern EDC platform.
User-friendly
User focused, with a track record of successful implementations in real-world clinical trials.
Highly customizable
Adaptable to extended user needs and workflows through configuration or plugins.
Modern, fully featured
Built on a modern stack utilizing the best design practices that make it easy to jump in and make modifications.
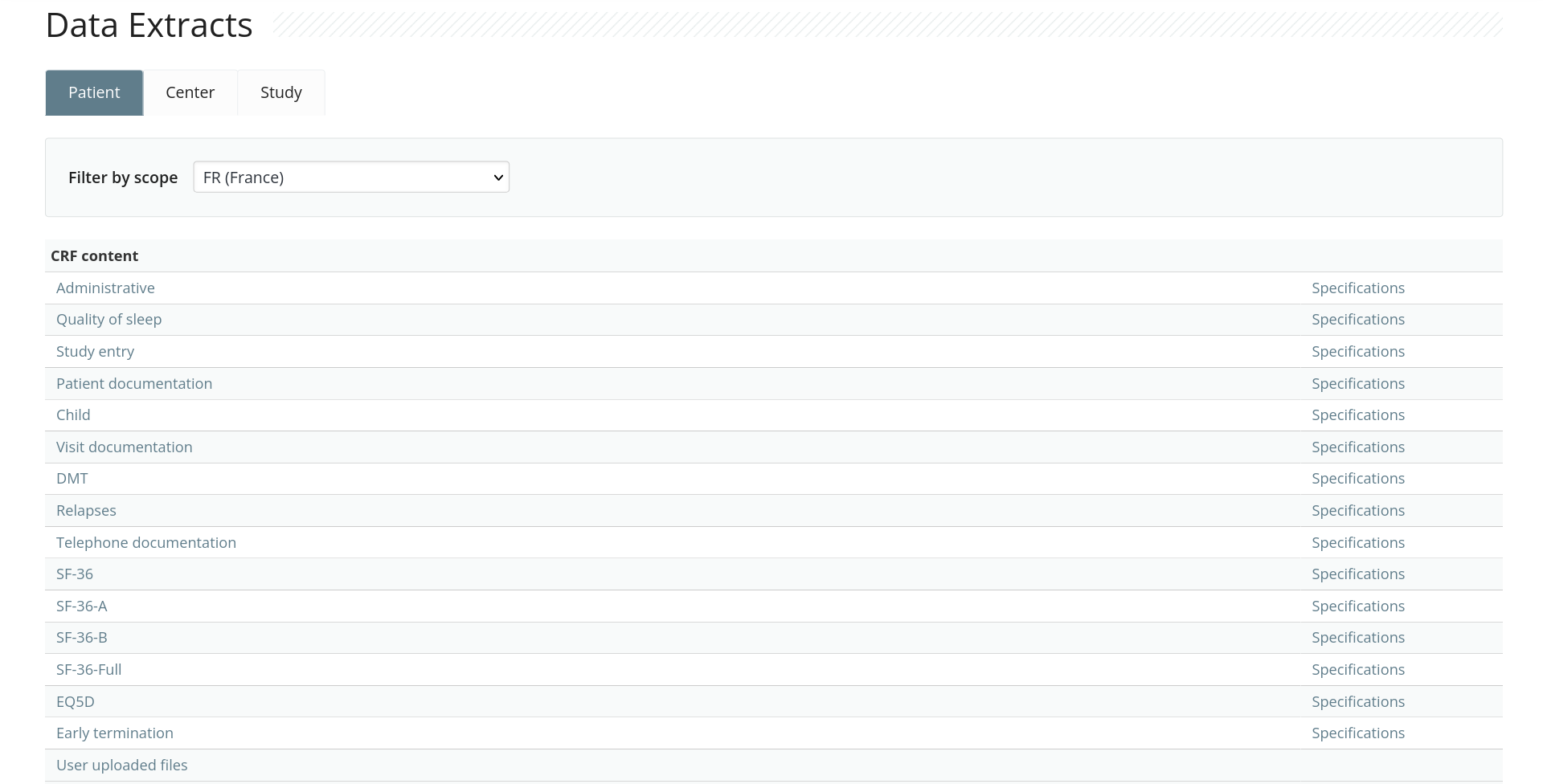
Interconnectable
Integrates with other systems through an HTTP API and an R SDK.
Easy to deploy
Fully dockerized for easy setup and deployment.
Free & Open-source
Rodano is 100% free and licensed under a AGPL-v3 license. All of our code is completely open source as well.
Compliant
Compliant with all the standards: 21 CFR Part 11, ICH GCP, GDPR
Interested? Let's get started!
Sponsors
This project would not be possible without the support of our sponsors:


